
Under the Mountain
He was pinned in the darkness by the weight of beams and walls, ice and earth. What happened? Where am I?
Lying on his stomach, he surveyed what he could. His left leg had been twisted and thrust forward so that his foot rested near his cheek. He could move his left arm, but his right arm and leg were crushed beneath something enormous. He realized with horror that his chin rested on the knee of a corpse. He tried to still the panic, to recall the moments before everything went dark.
He had been speaking to his wife. They were standing in a doorway. And then, the whistling gust of wind; the sense of tumbling through space; the sounds of coughing, moaning; and the horrifying silence that followed. Had it been an earthquake? He called to his wife but heard no answer.
“Giampaolo? Giampaolo?” a woman cried. She was close, and she was trapped here, too. “Are you alive?”
“I’m alive!” he shouted. “I’m alive.”
Nestled on the flanks of Italy’s Apennine Mountains, about 160 kilometres northeast of Rome, the Hotel Rigopiano had never been easy to reach. But its isolation only added to its appeal, attracting Italian pop stars and celebrities like George Clooney.
In January 2017, snow began to fall across the Apennines. For days it came down, and the enormous drifts ringing the Rigopiano grew taller by the hour.
From his home in the Rome suburbs, Giampaolo Matrone watched the weather with growing concern. He and his wife, Valentina Cicioni, had planned an overnight getaway to the hotel, but now he wondered whether they should go. Matrone phoned the Rigopiano. Its owner, Roberto Del Rosso, said Matrone simply needed chains on his tires. “Tranquillo,” Del Rosso said. “It won’t be a problem.”
What Del Rosso kept to himself was that things were growing bleak. Food and supplies at his hotel were running low, and with only a single snowplow, officials in town were struggling to keep the road open. But Matrone and Cicioni didn’t know that. They decided to make the trip. By the time they neared the resort six hours later, they were battling a total whiteout. When they finally reached the hotel, they were cold and exhausted. They checked into their room and then headed outside to the thermal pool, trying to forget their long day in the car. The wind and the thick, wet snow were unrelenting. Soon they retreated indoors and got dressed for dinner.
The conversation there was troubling. “I’m more scared than you,” a waiter confided to them. “I’ve been stuck here for six days.”

As the hotel guests awoke on Wednesday, January 18, they discovered that their predicament had worsened overnight. The cars in the lot were invisible. The phone and power lines were down. Cellular coverage, always spotty on the mountain, had gotten worse.
Employees in the kitchen were trying hard to manage what was left of the provisions. For breakfast, they put out a few microwaved croissants, some marmalade and Nutella.
After breakfast, Matrone and Cicioni went to the Jacuzzi. Matrone sank beneath the surface. Suddenly, the hotel began to wobble. The windows rattled, and the water in the tub sloshed over the edges.
An earthquake with a magnitude of 5.7 had struck the mountain. Matrone had had enough. “Let’s get out of here,” he told his wife, dressing quickly. They headed to the parking lot, where others were excavating their cars. Fifteen minutes after the first earthquake, another tremor hit, this one measuring 5.6.
With a dozen vehicles freed, guests set off down the driveway. But when they reached the main road, the path was blocked by a two-metre-high wall of snow. Matrone climbed out of his car and scaled the drift. There was no road, just a glistening expanse of powder. He turned and yelled down to his wife, “We’re trapped!” They made their way back to the resort.
As the light outside faded, the 40 guests and workers searched for distraction. Adriana Vranceanu and her son huddled over a checkerboard in a playroom on the ground floor. She needed aspirin and had sent her husband, Giampiero Parete, to find some in their car. Meanwhile, their daughter shot pool with two other children down the corridor in the billiard hall.
A handful of guests sat in white upholstered armchairs and sofas. Matrone paced the reception area, anxiously discussing options with his wife. Del Rosso, the owner, was just around the corner, in the hotel’s library nook. Half a dozen of his employees milled about in the kitchen.
This was when the snow on the mountain began to slide.
They heard the avalanche before they saw it. As the wall of snow and ice tumbled downward, it compressed the air into a terrible low whistle. The avalanche gathered speed and size, grabbing rocks and trees and anything else in its way as it roared down the mountain.
With the force of 4,000 fully loaded Mack trucks, the snow slammed into the hotel at 100 kilometres per hour. Walls buckled. The avalanche thundered through the kitchen, killing the workers there. It tore into the alcove where Del Rosso was, then raced across the two rooms where guests sat sipping hot drinks.
The snow—and the weight of everything it had brought down the mountain with it—ripped the hotel from its foundation, collapsed it into a pile of rubble, and sent debris flying more than 90 metres away. When the tossing and tumbling came to a stop, those caught inside were left buried in the icy heap of rock and ruin. All was now still; everything had gone dark.
Fabio Salzetta, the resort’s caretaker, had been working in the tiny boiler hut about 27 metres from the main building when he noticed an eerie silence. He tried to throw open the door, but it wouldn’t budge. The little outbuilding was sealed in snow. So he pried away the window frame and wriggled outside. Standing on an empty snowfield, he gazed at a trail of sheer destruction—it was as if a giant rake had been dragged down the mountain, toppling beech trees, crushing cars, chewing up everything in its path.
Salzetta felt numb. Where was the hotel?
Then he saw the tip of the Rigopiano’s roof poking out of a pyramid of ice-topped rubble. The entire building had been bulldozed down the hill.
Salzetta noticed a figure stumbling across the snow: Giampiero Parete. Moments before the avalanche had struck, he’d gone to get aspirin for his wife from their car. Parete appeared disoriented, distraught. “My family is inside the hotel,” he wailed.
The two climbed inside Parete’s car. Finding a cell signal with Parete’s phone seemed to take forever. In fact, it took two hours before they finally spoke with the chief of the region’s alpine rescue team, Antonio Crocetta.
“We’re coming,” Crocetta promised.
“How long will it take?” asked Parete.
“Five or six hours.”
Darkness had set in, and Parete and Salzetta sat afraid inside the car, the motor running, the heat now blasting.
From his base in the hillside village of Penne, Crocetta alerted the military police and mobilized his unit: 14 men trained in rescue operations, including a surgeon, an anaesthetist, a dog handler, a search dog and two veteran alpine guides. Reaching the resort would mean trekking 10 kilometres up the mountain.
When the rescuers finally arrived, eight hours after Parete had talked to Crocetta, there was no movement anywhere—no human sound, just rubble. They did spot two headlights: Salzetta and Parete in the car.
“How many people are in the hotel?” a team member asked.
“About 40,” Salzetta replied.
“Can you give us an idea of where they might be?”
Salzetta offered his best guesses. As rescuers wrapped Parete in a thermal blanket and took him down the mountain on a stretcher, other members of the alpine team began probing with tools used to poke through snow to locate bodies. They found the first one after about an hour, buried under one and a half metres of snow. It was Gabriele D’Angelo, one of Salzetta’s colleagues. The maître d’ of the hotel’s restaurant was found buried nearby. Later, Del Rosso’s body would be discovered beneath the rubble that crushed him as his hotel disintegrated.
While the alpine team probed for corpses, Giampaolo Matrone lay in a coffin-sized pocket of air beneath nine metres of snow, ice and rubble. He could hear nothing of what was happening at the surface. Shock had set in, and he felt no pain, no hunger, no cold.
He began to fade in and out of consciousness. Surreal imagery drifted through his mind. At one point, he was walking alongside his wife toward the bakery his family operated, taking note of every shop, every street corner, each crack in the pavement.
His thoughts were strange and richly detailed. He imagined rescuers swooping in on magic carpets, dressed like Aladdin in The Arabian Nights. In another vision, his best friend, a bodybuilder, materialized on the mountain, lifting tons of concrete and setting Matrone free.
Each time Matrone awoke, he confronted anew the terrible reality: he was buried alive. Despair washed over him. He asked himself, Who is going to save us?

The next morning, Thursday, more rescuers arrived, including a team of firefighters and canine trainer Lorenzo Botti. Looking at the remains of the hotel, Botti made a quick assessment: no chance of survivors.
Police technicians set up an antenna that allowed them to home in on buried cellphones. Wherever there were phones, there would be people—or, more likely, bodies.
Botti turned his attention to the nine-metre mound, buried under snow, that constituted the main structure of the hotel. After studying a crude floor plan drawn by Salzetta, Botti and his men mounted the wreckage, shovelled through four metres of snow, and, when they found the top of the building, began sawing into the roof.
Rescuers carefully lowered themselves through the apertures they’d cut. Lights fastened to their helmets illuminated the twisted debris. The space was so cramped, the rescuers had to crawl on their bellies. For hours they called out for survivors.
Finally, at 11 a.m. on Friday, more than 30 hours after the search began, they heard something astonishing: a woman crying for help.
“Who are you?” one of the rescuers screamed back.
“I am Adriana.”
“How many are you?”
“I’m in a room with my son,” she said. “My daughter’s inside, in the next room.”
Adriana Vranceanu—whose husband had gone to fetch her aspirin from their car before the avalanche and then frantically phoned for help—was bleeding from a head wound when firefighters found her and her son squeezed together in a crawl space. They were led to safety.
Finding the survivors electrified the rescuers. The firefighters tunnelled quickly toward the nearby billiard room, cut a small hole in the roof, focused a searchlight, and lowered a video probe. Gathered around a screen, the team saw two small kids emerge from behind a sofa, including Adriana’s daughter, drawn by the light from the hole in the roof. Somehow, the entire room appeared intact.
Through a hole in the wall, firefighters found three more children. “Stay calm,” a rescuer said. “Get on your bellies. Make like a centipede.” Carefully but quickly, the men ushered the children out of the rubble.
Meanwhile, another squad of rescuers picked up a cellphone signal coming from a crawl space. They sawed toward voices crying for help. Soon they found a young couple and a young woman, all of whom they pulled to safety.
“Who else is down here?”
“A Roman guy,” one of them said. “Giampaolo Matrone.”

It was after midnight now, Saturday, January 21, some 55 hours since the avalanche. The rescue team had been working nonstop for more than two days. The wreckage was frighteningly unstable. As they moved, the rescuers knew that the force of their digging—or another tremor—could bring it all down upon them.
Paolo Di Quinzio and three other rescuers burrowed on, breaking blade after blade on their circular saws, battling toward a faint cell signal detected deep in the ruins. Suddenly they heard a voice. They silenced their saws and listened. It was Matrone.
He was still fading in and out of consciousness. A vision of his wife, Valentina, hovered over him, an angel of mercy, he thought. She assured him he would be OK.
“Giampaolo, we are here!” Di Quinzio shouted, three metres above where the trapped man lay. “Are you injured? Are you bleeding?”
As the voices and the buzzing of saws grew louder, Matrone became more alert. “Where is my wife?”
“We put her in the car because it’s cold,” Di Quinzio said.
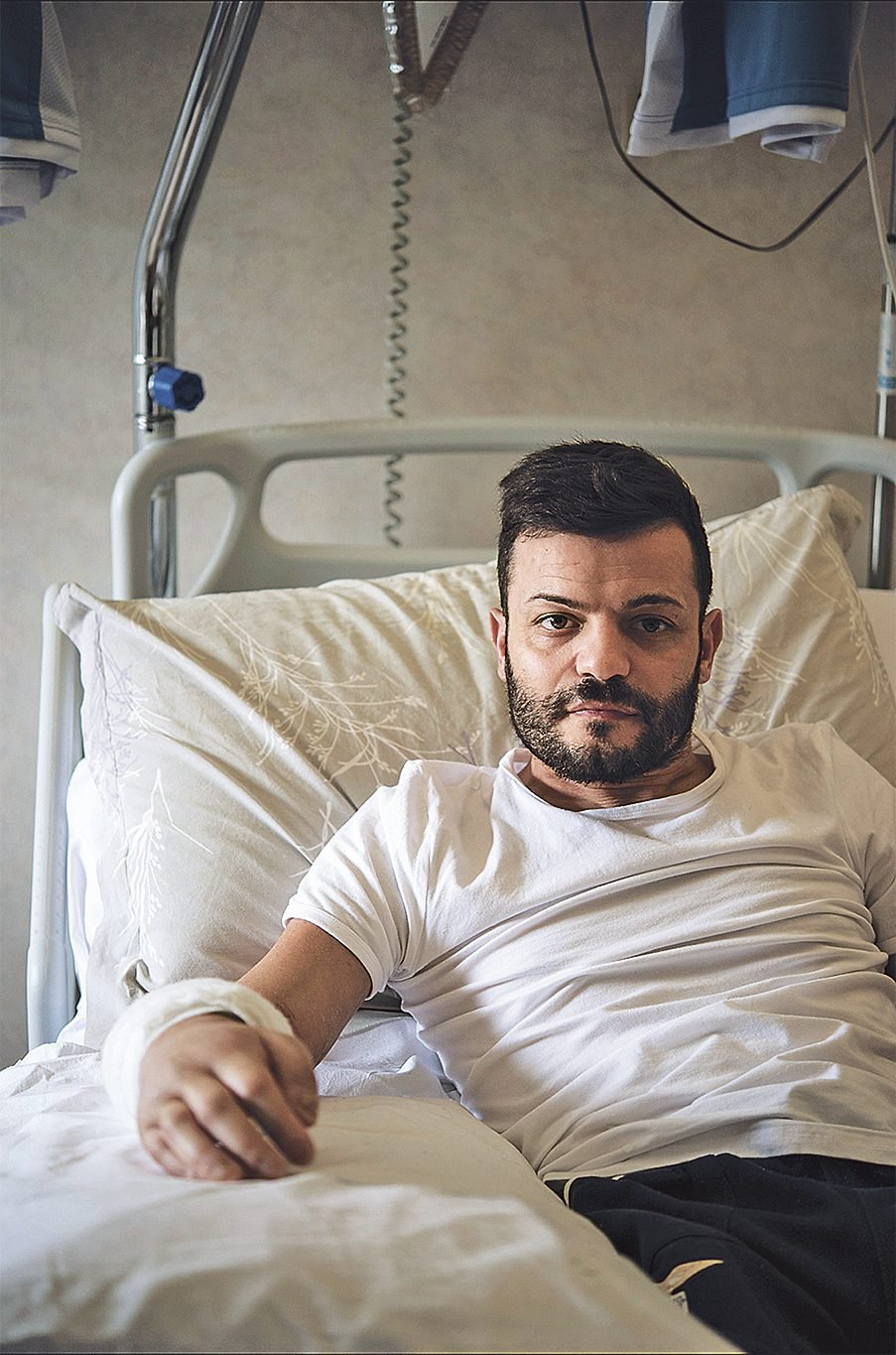
At last, at around six in the morning, Di Quinzio’s saw broke through a final thick layer of insulation. He pointed his light toward the opening and spotted Matrone’s back. Di Quinzio could see how the angled beams had created a cocoon that prevented Matrone from being crushed to death. Those near him had not been so lucky: squeezed in the space with him were the bodies of two women—one supporting his head, one curled beneath his left leg.
Rescuers raised the concrete beams off Matrone’s limbs with a hydraulic jack. “You are a superhero,” Di Quinzio said as he reached beneath Matrone’s armpits and gently lifted him out of his tomb.
The rescuers placed him on a mattress. Matrone gazed up at a dozen faces silhouetted by the light of their headlamps. A cheer went up in the small crowd: “Bravo!” He was one of 11 people out of 40 to have survived. Soon after, he was airlifted to a hospital in a nearby town.
Gangrene had begun to burrow deep into his right arm. The nerves in his right ankle had practically been destroyed. Had he been pulled out even two hours later, Matrone was told, his arm would certainly have been lost.
Five days after his rescue, Matrone was given the heartrending news that his wife had died. Her body had been found, crushed by debris, near where Matrone had been trapped. The angel who had appeared to him in his fitful dreams had never left his side.
Next, check out our collection of the most gripping survival stories.
GQ (July 2017), Copyright © 2017 by Joshua Hammer, gq.com.
Just as traditional as the Thanksgiving turkey is the overstuffed and satisfied sleepiness that follows. And although society has historically pointed a finger at turkey, it turns out that it’s not the main cause of your post-feast fatigue.
Why blame turkey for the fatigue in the first place? In a word: tryptophan. This chemical is an essential amino acid that is a component of the feel-good chemical serotonin as well as a precursor to the sleep-inducing hormone, melatonin.
Your body doesn’t produce tryptophan naturally so you have to get it through the food you eat.
Turkey is one of the foods famous for being high in tryptophan which is why it’s so often blamed for the post-dinner crash during the holidays. But that reputation may be overrated, says Darren Scott, PhD, a food scientist at Oklahoma State University.
Tryptophan can be found in all kinds of foods, ranging from dairy products and nuts to meats and tofu. And not only that, but turkey doesn’t have higher levels of tryptophan than any other common meat, he says.
In fact, gram for gram, even cheddar cheese contains greater amounts of tryptophan than turkey. So if the tryptophan in turkey really did cause our post-Thanksgiving drowsiness, we’d experience the same strong, lethargic sensation every time we ate chicken, beef, cheese, or nuts.
And, as we know, this obviously isn’t the case.

But if the turkey’s tryptophan isn’t to blame for our sleepiness, what is?
Holiday dinners are both heavy in the amount of food consumed and in the types of foods traditionally served.
Both of those things can contribute to the infamous food coma, Scott says.
Culprit No. 1: What you eat
Did someone say mashed potatoes, pies, stuffing, cornbread, green bean casserole, fresh rolls, and yams covered in marshmallows?
“Lots of traditional [holiday] foods are high in sugar and simple carbohydrates, which contribute to drowsiness,” he says.
The combination of protein and carbs may be particularly sleep-inducing, according to a study published in Obesity Research. Eating carbs causes the body to release more insulin into your blood, which increases amino acid absorption, including tryptophan. The extra tryptophan then increases the amount of serotonin in your brain. Serotonin, in turn, is a precursor to melatonin, the hormone that makes you feel drowsy.
The study also found that eating lots of sugary carbs can affect your mood in other ways, increasing feelings of depression and anxiety.
Culprit No. 2: What you drink
Alcohol is another reason your eyelids may grow heavy, says Amanda Kostro Miller, RD, a licensed dietitian/nutritionist who serves on the advisory board for Fitter Living.
On Thanksgiving, many adults drink beer, wine, or cocktails throughout the day and with their meals. Alcohol is a central nervous system depressant with fast-acting sedative effects.
It’s also high in quickly digested carbohydrates and may reduce your inhibition, causing you to eat more throughout the day, she adds.
Thinking of choosing punch instead?
“Sugar-sweetened beverages, including soda, fruit juice, sparkling ciders, sports drinks, sweet tea, and coffee drinks can also make your blood sugar spike and then drop, making you ‘crash,’ ” she says. “This up and down can not only affect your energy levels but it can also make your mood fluctuate.”
Culprit No. 3: How much you eat
Another factor may be the amount of food you eat over the holidays, Scott says. The average festive meal contains 229 grams of fat and 3,000 to 4,500 calories, according to estimates by the Calorie Control Council.
That’s more than most men and women eat in an entire day. Digesting all that food requires a lot of energy, so the body sends more blood to your digestive system to manage the load.
This means your body has less energy for other activities, like doing the post-meal dishes, and that makes the couch look even more appealing.
How to avoid the post-holiday dinner food coma
If you swear that you feel particularly sleepy after your holiday meal, it’s true—you’re not imagining it. But don’t blame the turkey.
If you don’t want to snore on the floor after you’ve cleared your plate, cut back on the sugar, carbs, and booze.
Try not to load your plate; eat moderate portions—you can always have leftovers tomorrow, Kostro Miller says.
Next, check out 11 leftovers that can make you sick.
Reader’s Digest Canada: Can people safely gather to celebrate the holidays this year?
Infectious disease specialist Dr. Isaac Bogoch: I love the approach of “how can we do this safely,” and I still think that’s the right lens to view many aspects of the pandemic. But when it comes to the upcoming holiday season, we have to be very careful about getting together with family and friends, because the rates of COVID-19 are so high across many parts of the country. As we’ve seen before, there’s going to be different rules in different jurisdictions about how many people can gather together. But we should really only be getting together with people who live under the same roof—and not have other people come into the home. I think we’ll start to see more messaging around that as the holidays get closer.
Did Thanksgiving in Canada teach us anything?
Yes. There’s been a pretty impressive rise in cases since then, and some of that is very likely to be attributed to activity around Thanksgiving. Certainly Christmas, Hanukkah, New Years and other holiday gatherings have the potential to amplify COVID-19 in many communities across the country.
If we don’t do big family gatherings this year, what’s going to be the payoff?
At the individual and family level, you really ensure that you have a safer holiday season and don’t put people in your family at risk, especially those who might be at risk of having a severe infection—like elderly parents or grandparents, or people who have underlying medical conditions. At a community level, you’re helping to prevent the amplification of this infection.
Imagine one household where COVID-19 ends up being transmitted among several people—and then they have friends or family over. Now magnifying that across the 38 million people in Canada… That has the potential to significantly spread this infection.
Some people are talking about taking their kids out of school, spending a couple of weeks being extra careful and then getting together with grandparents for holiday celebrations. Does that sound reasonable to you?
I have a couple of thoughts about that. For one, people don’t always recognize the number of contacts that they actually have in a day—while going out, getting groceries and going about their day. We actually have a pretty significant amount of contacts per day, as much as we’d like to think we don’t. However, if you can truly quarantine for 14 days—and really, truly not have any interactions with other people for that time period—then yes, from a medical and scientific standpoint, you wouldn’t pose any risk to other people. You might not be adhering to the local rules, but you won’t pose a risk.
Is there any other way to get that sense of togetherness this year?
We can find creative ways to connect with those we love through other channels, like phone calls and virtual get-togethers. As always, outdoor environments are much safer than indoor environments. It’s unclear what policies will be in place around outdoor gatherings when the holiday season rolls around, but if it’s allowed, I think it’s wonderful for people to get together for walks in the park. If you’re practicing appropriate physical distancing measures and wearing a mask, you certainly can have some social engagement with friends and family in smaller groups outside.
Can you update me on what we know about outdoor transmission?
It’s not impossible to get infected in outdoor environments but it seems to be exceedingly rare. A lot of situations have to line up for that to happen. Mainly, a pretty contagious person has to be in close proximity with you. Then, it’s gross to think about, but if that person with the virus coughs or sneezes right in your face, you’re probably going to get COVID-19. But fleeting contacts—like if you’re walking by somebody and they have the infection—are not how this virus is transmitted. So if you go for a walk with somebody and you’re outdoors and separated by a couple of metres, it would be very unusual to get this infection. We just don’t see amplification of the virus in outdoor environments.
So maybe small family groups might get together for winter activities like tobogganing?
Exactly—it’s great to do an activity that checks off a lot of boxes at the same time: get some fresh air, some ultraviolet rays, social interaction, physical exercise and mental stimulation.
If you do meet up with extended family outside, can you hand over a gift safely? We know transmission from touching things is low, right?
Yes, we now recognize that transmission from inanimate objects—called fomite transmission—is not nearly as common as it was once thought to be. It’s still possible, though, so if you exchange gifts it’s a good idea to wash your hands afterwards.
Next, find out when we can expect to get the new COVID-19 vaccines in Canada.
For a transmission to perform correctly, several electronic, hydraulic and mechanical systems need to work together seamlessly. When one or more of these systems fail, reverse is usually the first gear affected. Before panicking, let’s look at the issues preventing your car from shifting into reverse.
First, Automatic Transmission vs Manual Transmission
Automatic transmissions and manual/standard transmissions accomplish the same thing—a transfer of engine power and torque to the drive wheels. When shifting into reverse, the reverse gears interconnect with each other, then interlock with the transmission output shaft, ultimately turning the drive wheels.
When the driver of a vehicle with an automatic transmission shifts into reverse, a sophisticated pressurized hydraulic fluid system “automatically” activates an internal clutch pack and band that locks or unlocks reverse gearsets to each other and the output shaft. The driver of a vehicle with a manual transmission locks and unlocks the reverse gearsets by physically moving the gears using the shifter.
Possible Reasons Why Your Car Won’t Reverse
The drivetrain (the transmission and differential) is how engine power gets to the wheels. Various transmission and differential components and gears multiply engine power and then divide it between the wheels. The fascinating thing about engine power is, without friction holding the tires to the road, there is simply no way to harness that power. Here are the most common causes your car won’t go into reverse.
Transmission fluid levels
A transmission low on fluid will cause the engine to race but not shift into reverse. Check your owner’s manual to locate the transmission dipstick and the specific automatic transmission fluid (ATF) or manual transmission fluid your vehicle’s make, model and year needs.
AFT levels should be between the “full” and “add” marks on the dipstick. Add new ATF using a clean funnel if it’s low. If your car does go into reverse after adding ATF, your next stop should be your mechanic to check for leaks. It may be a good time to flush the ATF and replace the filter.
On a manual transmission vehicle, it will most likely need to be jacked up so the transmission fill plug can be removed and the fluid level checked. Leave this to your mechanic if you’re not comfortable crawling under your car. Find out how to check all car fluids on your own.
Shift mechanisms
Many vehicles with an automatic transmission have an electronic Transmission Selector Switch that signals the computer (ECM). The ECM then signals other sensors and components that ultimately engage reverse gear. Moving the shifter slowly from Park to Low several times can help remove corrosion from the switch’s contacts and get you on your way. Leave transmission electronics diagnostics, internal repairs and shift cable replacement to transmission specialists.
On a manual transmission vehicle, misadjusted shift linkage and damaged or stretched shift cables can keep the reverse gearset from locking when moving the shifter. With an automatic transmission, bad cables or misadjusted linkage can make the shift indicator point to Reverse when you’re actually in Neutral. Check your owner’s manual; some shift cables are adjustable. Adjusting a cable is a one-time fix you can make before taking your car in for service.
BE AWARE: Automatic transmission vehicles feature a park-neutral safety switch that prevents starting in reverse or drive. A cable too far out of adjustment will permit a car to start while in gear, causing it to lunge forward or backward.
Transmission, gaskets, seals, O-rings
External transmission gaskets, seals and O-rings are designed to keep transmission fluid in the transmission while keeping contaminates out. Dirty or contaminated ATF cannot properly lubricate internal parts. This results in an overheated transmission that causes sludge and varnish buildup.
Sludge buildup causes premature gear and bearing wear and gasket/seal failures. Leaks usually appear in the pan (automatic transmission) or side cover gaskets (manual transmission) and output or drive axle shaft seals. On an automatic transmission, leaking internal gaskets, seals or O-rings will keep clutch packs and bands from engaging gearsets—a common cause for the inability to go in reverse. Leave these for your mechanic to fix.
Valve body
Truly a marvel of modern engineering, an automatic transmission valve body contains several electronic and fluid-activated mechanical shift valves that supply hydraulic pressure to the clutch packs and bands that engage one or more gearsets. Valves binding from an overheated transmission, a failed valve body gasket, clogged hydraulic passage or leaking shift solenoids will keep your car from going into reverse. Diagnosing and repairing valve body problems is best left to an automotive transmission specialist.
Broken gears
Damaged gearsets are likely to produce a grinding or gnashing sound when shifting. (Here are more strange car sounds you should never ignore.) A stripped-out automatic transmission reverse gearset or broken manual transmission reverse idler gear will keep a vehicle from going into reverse, but will not keep your car from driving forward.
Besides the obvious noise of gears crunching against each other, metal-to-metal grinding generates metal dust and chips that will damage other transmission parts. Leave transmission repairs to the pros. To prevent future transmission problems, insist your mechanic thoroughly flushes the transmission case, valve body and radiator cooler with solvent as part of any transmission repair.
Other possible causes
First, is the parking brake fully released? Sorry, had to ask. (Find out nine times you should be using your emergency brake.)
Parking on a steep incline can jam the lock pin against the gears that keep your car from rolling away when in Park. This makes it difficult to move the shifter. Rocking back and forth in the front seat should release pressure off the pin.
In cold temperatures, let the car warm up before shifting into reverse again. Transmission fluid may need to heat up before it flows properly (or warm up a hardened seal) especially when it’s cold outside. With a manual transmission, try pumping the clutch and shifting into first and second gear several times before attempting to shift into reverse to free up a stuck clutch plate.
IMPORTANT! Always be in the driver’s seat with the shifter in “Park” or “Neutral,” your foot firmly on the brake and parking brake set before attempting to start your car or move the shifter. Never reach into your car through an open door or window from outside the car to start the engine or move the shifter into gear. Even with the engine is off, placing a car in gear (either accidentally or on purpose) will cause it to lurch forward or backward, which can cause serious injury or death.
The Last Word
Be kind to your transmission. Come to a complete stop before shifting. Don’t race the engine in neutral and then shift into drive; you risk damaging your transmission. Performing regularly scheduled maintenance and flushing ATF every 30,000 miles (48,000 kilometres), will give you many years of trouble-free service.
Next, find out the most common car problems—and how to fix them yourself.
On November 9, U.S. pharmaceutical company Pfizer announced that its mRNA vaccine, which is being developed with German biotech company BioNTech, appears to protect against SARS-CoV-2, the virus that causes the COVID-19 infection.
Seven days later, Massachusetts-based Moderna reported that, according to preliminary findings, its own mRNA vaccine may be up to 94.5 per cent effective.
Then, more news: Pfizer gave an update that its vaccine had reached stage 3 clinical trial efficacy points. It concluded that its vaccine is 95 per cent effective and is beneficial to older people. The company said it would be applying to the U.S. FDA for emergency use authorization “within days.”
The back to back announcements have naturally generated considerable excitement. But it’s probably wise to leave the champagne corked for now. The findings are “promising,” says Susy Hota, an infectious disease specialist and the medical director of prevention and control at the University Health Network in Toronto. But there are still a few hurdles left to clear before the vaccines are made readily available.
Here’s what you need to know about these vaccines—and when they’ll be available to Canadians.
Are these COVID-19 vaccines approved by Health Canada?
Both Pfizer and Moderna began their clinical trials in late July of this year. Pfizer enrolled nearly 44,000 participants, Moderna 30,000 participants. Part of the inclusion criteria for participants was that they had an “appreciable” risk of contracting the virus either because of where they lived or other circumstances. Each set of participants were divided into two groups: people who were given two doses of the vaccine and people who were given placebo or saltwater doses of the vaccine. Each trial was evaluated by an independent board of experts.
Health Canada is reportedly working directly with pharma companies like Moderna and Pfizer, getting the trial results as they become available in real-time rather than waiting for the studies to conclude—a situation which could potentially shorten the time for eventual approval here.
If the Pfizer and Moderna vaccines are approved by the FDA for emergency use, Hota doesn’t think there would be a measurable lag between that and Health Canada approval.
“It has to go through Health Canada’s approval process, too,” she says. “But I don’t think that’ is going to be a lengthy, difficult process.”
What’s an mRNA vaccine, and how does it protect against the coronavirus?
Both the Pfizer and Moderna candidates are mRNA-based vaccines—a type of vaccine that uses genetic material to provoke an immune response against the virus in the body (rather than conventional vaccine formulations that use live or attenuated forms of the virus itself to fight infection).
RNA is a type of nucleic acid we already have in our bodies. The Pfizer and Moderna vaccines work by injecting an mRNA code that prompts the existing cells of your body to produce a replica of the spike protein that sits on top of the Sars-Cov2 virus. “Your immune system recognizes that spike protein as foreign and attacks it,” says Hota. The immune system produces an army of “soldiers” that are primed to attack that spike protein should it appear in your body again—as it would if you subsequently became infected with the virus.
An mRNA-based vaccine is an attractive option to fight the coronavirus because of its potency, its relative low cost, and its capacity for being developed and administered more rapidly in a lab than conventional vaccines.
There is an added wrinkle with the Pfizer vaccine, and that’s storage. Its vaccine needs to be kept in very cold conditions, around -70C. That presents an extra layer of challenge when it comes to distribution as that puts most pharmacies and doctor’s offices out of the running. By contrast, Moderna says its vaccine can be refrigerated at -20 C, a temperature that makes it more likely to be distributed conventionally in doctor’s offices and pharmacies.
How long do these COVID-19 vaccines protect you against infection?
The short answer: that’s to be determined. It’s not clear yet whether or not the vaccines offer lasting protection against the SARS-CoV-2 virus that causes COVID-19, or whether the immune response they generate lessens over time. It’s also not firmly established whether or not the vaccines benefit most adults broadly or just certain groups. In its latest announcement, Pfizer indicated its vaccine is beneficial to people over 65 years old, but the clinical data supporting that claim hasn’t been made readily available.
Time will also tell whether or not the vaccine offers protections to people who’ve had the virus before and if it can act to reduce the severity of the virus should someone get infected after being vaccinated.
Are they safe?
The preliminary findings in both trials suggest there are no significant safety concerns with either vaccine. That said, the data is still being collected and both companies intend to follow trial participants for a period of years.
That long-term follow-up is standard procedure with any new medication or therapy, says Hota. She doesn’t see any red flags when it comes to safety but warns that the public should be primed for a few bumps in the road ahead. “It’s likely that over time we will learn which vaccines are better than others and that will be a longer process,” she says.
The vaccines do appear to generate some side effects, including headache, soreness and fatigue. These kinds of side effects aren’t uncommon: the shingles vaccine can cause similar symptoms.
When will they be rolled out across Canada?
That depends on when they’re approved by Health Canada. Prior to Pfizer’s update, Prime Minister Justin Trudeau had said he believes a vaccine could be available in Canada during the first three months of 2021. It’s not clear if higher risk populations and health care workers in Canada would be given the vaccine earlier than that, but it’s fair to assume they would be first in line.
Canada has reached a deal with Pfizer to buy 20 million doses with options of up to 56 million additional doses (a total of 76 million doses). The government has a similar deal with Moderna for 20 million doses with options for up to 36 million additional doses (a total 56 million doses).
These two aren’t the only vaccines Canada is eyeing for use here.
A representative for the Government of Canada’s Public Services and Procurement Department, which deals with the vaccines, confirmed that Canada currently has agreements with seven different companies, including Pfizer and Moderna.
What does this mean for us right now?
Aside from the much-needed mood-booster, not much more than a TBD “Save the Date.” Canada and much of the rest of the world is still in the middle of a pandemic and infections are growing exponentially. Canada currently has over 300,000 reported cases of coronavirus.
We’re in for a long winter, and this is not the time to let your guard down. If you want to pop that champagne cork sometime in 2021, you’d be well advised to continue to socially distance, reduce your contacts and wear a mask.
“These public health measures are more important than ever now,” says Hota.
Next, find out the science behind the “super-spreaders” of COVID-19.

Our best shot
The sprawling Medicago facility in suburban Quebec City smells like a botanical garden and sounds like an airplane hangar. Thousands of Nicotiana benthamiana plants, a close cousin of tobacco, grow in long rows amid noisy ventilation. When the plants are six or seven weeks old and maybe 20 centimetres tall, they’re lined up by the dozens onto a flatbed that’s then inverted over a tank filled with fluid. The plants get dunked; the tank seals; and the leaves begin to absorb the liquid, made of a bacteria that’s been slightly tweaked. Bits of its DNA have been swapped out for DNA from the spike protein of SARS-CoV-2, the virus that causes COVID-19.
Once the plants come out of the tank, they’re moved to an incubation chamber. For the next week or so, the bacteria will insert its genetic information into the plants, triggering the production of millions of spike proteins in every cell of the infected leaves. The spikes self-assemble into something called a virus-like particle—not the virus itself but a particle roughly the size and shape of SARS-CoV-2. Gowned workers come and harvest the plants, stripping the leaves like they’re plucking basil for pesto, then send them to be shredded.
The chopped-up leaves head next into a vat of enzymes and are left to soak overnight. The enzymes work to break apart the cell walls, releasing the virus-like particles so they can be collected, purified and converted into a yellowish vaccine. This doppelgänger for SARS-CoV-2 can’t inflict any real damage. “But when you inject it into someone, the immune system sees it as though it’s the real virus and thinks, Oh my God, there’s an invader here,” says Medicago executive Nathalie Landry. “And then it will trigger a good immune response.”
A vaccine is, in essence, a trick—a sleight of hand that convinces your body to mount a counterattack to a given pathogen before that pathogen actually infects you. There are various ways to pull off the trick: vaccines can be made with a weakened virus, or a killed virus, or just a key part of the virus, or a part of the virus piggybacking on a different, benign virus, or the genetic instructions to make that key part of the virus yourself. In each approach, you get the benefits of an immune response without the messy business of a disease.
An effective vaccine is a crucial tool for combatting a virus impervious to borders, seasonality and many of the lockdown measures employed by anxious nations. But it’s not the pandemic finish line—it’s more like a pandemic off-ramp. Winding epidemiological, logistical and ethical roads still lie ahead: to determine how long and how well that vaccine’s protection can last, to manufacture enough of it to jab into billions of arms, to allocate the first batches of supply between countries and within their populations and to persuade vaccine skeptics to roll up a sleeve. Do that and we can protect the entire planet—all 7.8 billion of us.
Vaccine history
Humans have been trying to outsmart viruses for millennia. By the late 1600s, Chinese doctors were grinding a smallpox scab into a powder and blowing it up a healthy patient’s nose. An ambassador to Britain sent reports of 17th-century North African surgeons making a small incision between the thumb and forefinger, then squeezing smallpox pus into the wound. At the turn of the 19th century, Edward Jenner extracted fluid from a milkmaid’s cowpox blister and scratched it into the arm of an eight-year-old boy. These efforts may seem crude now—we prefer our vaccines packed tidily in glass vials and injected through sterile hypodermic needles—but the idea remains the same: teach the immune system how to ward off a virus so it has a head start should infection occur.
When a new pathogen invades the human body, our innate immune system recognizes the presence of something noxious and sends up an alarm. The first responders are proteins that meddle with a virus in order to limit its ability to reproduce. After that, the adaptive immune system kicks in: B cells (a type of white blood cell) begin making antibodies, the proteins that can subdue a virus by blocking its ability to get into the body’s cells. T cells (another type of white blood cell) arrive with two purposes: to help B cells make more antibodies and to assassinate cells the virus has already infected. It’s a more sophisticated response, but it’s also slower, taking a week, sometimes longer, to mobilize, which might not be soon enough to stop a virus from commandeering your body.
If the infection is cleared, many of the body’s B and T cells then die off themselves. Some, though, transform into memory cells, typically bunkered down in your bone marrow, where they wait to spring into action the next time that same pathogen attacks. “All it takes is one B cell to recognize the target and get activated, and it will start proliferating so it can produce the antibodies,” says Marc-André Langlois, a molecular virologist at the University of Ottawa. “That’s why vaccines work. They give you this life-saving element of having the antibodies ready to be deployed.” A defence that would otherwise take the body weeks to mount can be summoned in just a few hours.
The different types of vaccines
We’ve come a long way from smallpox pus, but to develop a vaccine, scientists still need to pick their poison. In modern medicine, that decision involves choosing whether to use the entire virus or just a vital part of it. Whole-virus vaccines take the pathogen—grown in giant batches of chicken eggs or in cells—and then kill it, usually with heat, chemicals or radiation. The upside is that the virus doesn’t cause disease once it’s introduced to the body, but it also doesn’t always cause a strong immune response, often requiring multiple doses. This approach is used in the flu shot and a hepatitis A vaccine, as well as in the one for polio, which a global vaccination effort has essentially wiped out.
In a more recent variation on the whole-virus vaccine, a pathogen is weakened in a lab rather than killed outright. Chances are you’ve been jabbed with a bunch of these vaccines: they’re how we fight measles, chicken pox, yellow fever and tuberculosis. Because the vaccine closely resembles a natural infection, it typically elicits a robust, enduring response. Because the vaccine is more potent, though, people with compromised immune systems are often unable to get it at all.
Another strategy is to block part of the virus rather than the whole thing. There are several ways of introducing this target protein—which is called the antigen—to the body. Most of them require another ingredient, usually aluminum, which for the past 90 years has been added to many vaccines to boost the immune response; aluminum stimulates our dendritic cells, which help trigger the adaptive immune system.
One way is to be directly injected with the pathogen’s critical protein, which triggers an antibody response. Virus-like-particle vaccines, such as Medicago’s plant-grown candidate, are a type of these protein-based vaccines. It’s a proven method—HPV and hepatitis B are just two examples—and there are usually few side effects once you’re given the shot.
Then there are genetic vaccines, which don’t deliver the antigen itself but instead issue a blueprint of that target protein to our bodies, hijacking our own cells to produce it. In a DNA vaccine, DNA containing the gene for the antigen is delivered to the cells. The cells copy those genetic instructions into molecules called messenger RNA (mRNA), which issue marching orders to the body to assemble the antigen. Messenger RNA vaccines skip the DNA entirely and deliver mRNA straight to the cells. The production time for such a vaccine can be cut down dramatically, which is why some researchers believe a genetic vaccine for COVID-19 will be ready first. But this is uncharted territory: no DNA or mRNA vaccines are currently approved for human use.
SARS-CoV-2 is a stealthy operator. It has a gift for binding to receptors on cells scattered in high numbers along the lining of our respiratory tract. When contact is made, it creates an opening through which the virus can pour its genetic code, the RNA, into our bodies. “The moment the RNA enters the body, it takes over the cell—there’s no wasting time,” says Natalia Martin Orozco, vice-president of drug development at Toronto’s Providence Therapeutics, which is working on an mRNA vaccine for COVID-19. The first proteins that SARS-CoV-2 produces are not to make more copies of itself but instead to suppress an immune response.
There are now more than 200 vaccine candidates for COVID-19 in development around the world, using every conceivable approach. Not all of these candidates, in the end, will work—many of them won’t. And it’s not yet clear what exactly will ward off this virus. Immunity isn’t an on/off switch: there are multiple levels of protection conferred by either shaking off a disease or receiving its vaccine. Some vaccines, like the one for hepatitis A, provide sterilizing immunity, which prevents the infection and its transmission almost entirely. Others, like those for diphtheria and tetanus, generate neutralizing immunity, where an infection can occur but won’t get very far and can’t make someone sick. Often protection isn’t lifelong, so we need booster shots to shore up our immunity.
When it comes to coronaviruses, immune responses tend to be short-lived: two to three years for the first SARS virus, for example, after which people exposed to that same pathogen would likely fall sick once again. Still, three years of protection sounds pretty good right now. As Martin Orozco says, “Even if the vaccine lasted just one season, that, to me, would be a really great accomplishment.” In the midst of a pandemic, a SARS-CoV-2 vaccine that performs as well as a flu shot is nothing to sneeze at.
Lessons from the flu
Twice a year, a consortium of scientists representing influenza centres in more than 100 countries descends on the World Health Organization (WHO) to pick the flu strains that should be combatted by seasonal vaccines. Once the recommendations are made, the viruses are produced in WHO laboratories, then shipped to the companies around the world that manufacture the corresponding vaccines. There is no centralized body whipping up batches of SARS-CoV-2 for developers looking to try their hand at a COVID-19 vaccine. Instead, they need the genetic code for the virus, which Chinese researchers sequenced in the second week of January and shared in a public database. Once scientists determined what was inside the 30,000 “letters” of this coronavirus’s RNA, they could decide which proteins to target in their vaccines.
After developers pick their antigen and their delivery system, they test it, starting with animals. Because ferrets and hamsters are, like us, naturally susceptible to SARS-CoV-2, they were a popular choice for early vaccine trials at the University of Saskatchewan’s Vaccine and Infectious Disease Organization-International Vaccine Centre (VIDO-InterVac).
If the vaccine protects those animals from infection, the next step is a safety trial with dozens of people to see if fevers spike or injected arms swell, followed by another trial, which measures the strength of the immune response that’s produced. Then it’s on to the third trial, where thousands of volunteers are monitored for a statistically significant difference between rates of infection in an unvaccinated control group and in people who actually got the jab. At least nine leading candidates have entered Phase 3 trials, including ones from the University of Oxford, Moderna and Pfizer. Currently, the WHO has set the minimum bar for an effective vaccine at an infection-reduction rate of 50 per cent, though 70 per cent is preferred.
What’s needed to make enough doses for these trials depends on the type of vaccine. For Medicago’s plant-based candidate, there must be well-stocked greenhouses and a dunking tank. To take another example: at VIDO-InterVac, where researchers are working on a protein-based vaccine, they begin with a single cell. “We take the gene from the virus that encodes for the spike protein and we put that gene into the single cell, which now thinks it is its own protein,” says VIDO-InterVac director and CEO Volker Gerdts.
At first, scientists use a flask that contains everything necessary to make a cell happy—some sugars, a couple of amino acids, a nice, warm environment and a little CO2—so the cell is fooled into believing it’s still in a body. One cell divides into two, then four, then eight, then 16; the flask becomes a three-litre beaker, the three-litre beaker becomes 20 litres, then 250, all the way up to a 1,000- or 2,000-litre bioreactor. From one individual cell you can make enough of the protein for thousands, even millions, of doses.
Making a successful vaccine is one challenge. Making enough of it to satisfy world demand is another. Every facility needs to conform to Good Manufacturing Practices (GMP), which are exceptionally specific rules set out by the WHO that ensure quality control. You want consistency over time so that each successive batch is the same.
Many Canadian labs can produce enough vaccine for their clinical research under these strict GMP conditions. But when it comes to scaling up production, we’re not in nearly as strong a position. There are two facilities in Canada with large-scale production capacity: the National Research Council, which partnered with CanSino Biologics to produce its vaccine and received a recent $126-million boost from the federal government; and Medicago, which signed a contract to supply the federal government with 76 million doses of its in-development vaccine, which if clinical trials go well could be ready for distribution to the public in the first half of 2021.
A third facility is slated to be built in Quebec, with greenhouses the size of two football fields, though that won’t be completed until 2023. And, in March, VIDO-InterVac received $23.3 million from the federal and provincial governments, half of which will be used to complete its own much larger facility, which should be ready by July 2022.
In the meantime, Canada has turned elsewhere for treatments. In early August, Procurement Minister Anita Anand announced the first of a pair of deals with American companies Pfizer and Moderna for tens of millions of doses of their vaccines. By October, Canada had made arrangements with four additional companies, committing a total of more than $1 billion for nearly 200 million potential doses.
Plenty of other countries inked deals of their own this summer. By mid-August, preorders of COVID-19 vaccine candidates were reportedly stretching toward six billion doses, the majority claimed by wealthy nations. But every country and company will be competing for the same limited supplies. Already there have been murmurings of glass shortages that could curb the availability of vials; stoppers are made by only a handful of companies. It doesn’t take much to cause a major bottleneck.
What happens after we have a vaccine?
We might be inventing a vaccine from scratch, but we’re not inventing a whole new system to get it into the arms of Canadians. When it comes to distributing vaccines for COVID-19, Canada will most likely take cues from the influenza-vaccination programs we have in place now. For those, Health Canada approves and then bulk orders the vaccines, choosing a couple of different candidates in case there are manufacturing snafus.
The provinces then determine how exactly to get the doses out, allotting a certain share to family doctors, public health clinics, community clinics and pharmacies. Typically they’ll also decide whether they will publicly fund vaccination and for whom. Ontario has a universal flu-vaccination program, for example, and British Columbia and Quebec do not, though it’s hard to imagine that anyone will have to shell out for a COVID-19 shot.
While flu shots are ordered and distributed based on how many people got one the previous year, planning for COVID-19 vaccines presents its own challenges: we don’t know what the supply is going to be, how well it will work in different populations or how many doses the vaccine might require. In fact, administering the flu shot this influenza season will be a good trial run for getting out a COVID-19 vaccine. Although physical-distancing measures and travel restrictions might mean a milder flu season, health-care officials in Canada are anticipating higher demand this winter. Expect longer hours, assigned appointments and perhaps even at-home vaccinations, especially for high-risk or vulnerable people.
The National Advisory Committee on Immunization (NACI), formed back in 1964 to review administering the polio vaccine, among others, makes recommendations on immunization practices and schedules, including which populations (often front-line workers and the elderly) should get the vaccine first.
NACI advises on priorities, but because health care is a provincial responsibility, it’s up to the provinces and territories to actually implement those recommendations. And it’s the provinces that actually set most of the disease-control goals. Do you vaccinate to prevent mortality? In that case, for this virus, the elderly need to be prioritized. Do you vaccinate to reduce transmission and spread? There are some house-partying twentysomethings in Kelowna who could get the jab. Or do you vaccinate widely in an attempt to achieve herd immunity?
We know, when it comes to COVID-19, that racialized and low-income people are infected at rates wildly disproportionate to their populations, not for any epidemiological reason but because of historical and economic disadvantages. This inequality persists for those employed in the health-care system itself: according to a study of almost 100,000 health-care workers in the U.S. and U.K. published in The Lancet, racialized workers were nearly twice as likely as their white colleagues to come down with COVID-19. Perhaps decision making about vaccine prioritization should be based on structural social causes instead.
It’s been a year since the virus arrived, and in that time, a few hundred vaccine candidates have been created, dozens have entered human trials and pretty much every promising new technology has been pressed into action. Work that would normally occur in sequence and stall on some bureaucrat’s desk is now, thanks to huge financial investments by governments around the world, happening swiftly and in tandem. That still won’t buy an end to this pandemic as quickly as we’d like: there’s much mask-wearing and social distancing and staying home ahead. But the average new vaccine takes about a decade to make it to market. The fastest ever to make it to market, for mumps, arrived in four years. We’re virtually guaranteed to shatter that record for COVID-19—one more unprecedented event in an era already full of them.
© 2020, Danielle Groen. From “How to Vaccinate a Planet,” from The Walrus (September 15, 2020).
Next, here’s what Canadians need to know about the new COVID-19 vaccines.
Even if you love fish, actually cooking it in your kitchen can be a commitment. Long after it’s been eaten, the smell lingers. It can be enough to put even the most devoted fish eater off cooking it regularly. Luckily, expert cooks from around the Internet have figured out how you can cook your fish without leaving behind a stench that lingers for days.
Fresh fish smells
First off, know that truly fresh fish really shouldn’t have much of an odour at all. So if you unwrap a package of fish and take a step back from the smell that greets you, toss it. But, admittedly, even the freshest fish can get a bit of stink going in your house once it’s cooked.
Methods for cooking the fish
A lot of the smell from cooking fish is because of the cooking method that you use. Frying can cause a massive odour problem, but other techniques, like cooking fish in foil or paper, can put a lid on the problem before it starts. Cleaning experts recommend neutralizing the odour before cooking by soaking fish in milk or a solution of lemon and water. (Here are the mistakes to avoid when cooking fish.)
How to get rid of the fish smell
If it’s too late and your home is beginning to smell like the parking lot of a seafood joint, then Good Housekeeping suggests mixing together vinegar and water and letting it boil for several minutes. You can also add cinnamon sticks or lavender oils for a more pleasantly scented atmosphere.
Prep your home ahead of time by shutting any inside doors to keep the smell from spreading throughout the house and into bedrooms, but open up kitchen windows and doors to allow the smell to ventilate. And be sure to wipe any spills and toss any trash containing fish bits as soon as possible to help prevent any lingering odours. When all else fails, bake up some cookies for dessert and the resulting sweet scent will make everyone forget about any fishy smells.
Sources:
- Epicurious: “The Quick Shortcut to a Better-Smelling Kitchen”
- Cleanipedia: “How to get rid of the smell of fish”
- Good Housekeeping: “5 Smart Ways to Zap Kitchen Odours”
Next, learn how to get rid of odours with these 20+ items in your pantry.
Our relationship with trees can be quite personal. The volunteers at the Highway of Heroes Tree Campaign have certainly found this to be true.
When we began five years ago with the goal to plant 117,000 trees on the Highway of Heroes—one tree for every Canadian lost at war since 1812—we had no idea just how personal the experience would become.
We discovered that 117,000 trees fit nicely along the Highway right-of-way, between CFB Trenton and the coroner’s office in Toronto. As word spread that we needed to raise ten million dollars to get the job done, volunteers and donors came out of the woods (pun intended). Members of Landscape Ontario, a strategic partner, have provided our campaign with donated trees, mulch, soil and labour—and many more Canadians have donated their hard-earned cash.
Over 4,500 amazing people also put their hand up to say, “I want to be a part of this.” We’ve had whole teams of junior hockey, baseball and soccer players, teachers and students, church groups and social clubs alongside people who stood in respect on the bridges during the Afghan conflict, local Legion members and active service personnel by the bus load, and of course, most powerfully, Silver Cross mothers (and fathers) coming together to plant trees together.
For decades, the seemingly ever-expanding Highway 401 was nothing more to Canadians than a ribbon of asphalt to be travelled on. That was until our nation deployed our troops to Afghanistan.
In all, 40,000 men and women from Canada’s Armed Forces served in the Afghan mission between 2001 and 2014.
Beginning in the region of Northumberland, Ontario, first responders began to gather to express their gratitude and grief, as 159 fallen Canadian heroes made their final trip home.
Soon after, many thousands of us joined them to pay quiet tribute to our fallen.

The Maple Leaf Forever
The Afghan conflict marked the first deployment of service personnel where the bodies of our fallen would be repatriated to Canadian soil. Now, it is our mission to honour their legacy.
We are planting two million trees for two million heroes. In addition to the 117,000 trees we are planting for our fallen heroes, we are planting over 1.8 million more trees in the communities along the entire 401 freeway—one for each Canadian who volunteered for service during times of war.
Our trees stand as a living tribute to those who gave their lives to protect the land they are planted on; they too continue to protect us and our land. The trees along the highway also become an integral part of our transportation infrastructure—blocking wind, drifting snow and preventing flooding.
By the end of 2020, we will be approaching the halfway mark of our two million tree goal. Inspired by yellow ribbons that are often tied to trees as a tribute to support those who serve, the trees we plant on the highway have all been given a yellow wrap at their base. The wraps not only protect the trees from rodent damage, but also make our trees easier to spot from the highway.
Off the highway, private landowners living within 30 kilometres of the highway along the entire 401 corridor have the opportunity to have a piece of this living memorial to call their own. Our Private Landowner Partnership Program, which would not be possible without an imperative partnership with Forests Ontario, supports tree plantings for qualified landowners at a substantially reduced cost.
In just over two years, our final tree will be planted, and our work will be complete, but we still need the help of all Canadians to get the job done.
First, please spread the word. Our campaign has the power to unite Canadians. We know that the more people who know about it, the more effective we will be at honouring our veterans, protecting our environment and expanding our natural heritage.
Also, until the end of this year, donations will be matched by an anonymous Canadian donor. For a donation of $150, you can help us plant a single tree, dedicated in the name of your choice and in honour of a Canadian veteran.
Generations United
Our natural heritage is not ours alone. We inherited it from our grandparents and the Native Peoples of Canada who stewarded the land before Europeans arrived. We have a responsibility to care for it today so we can pass it on to our grandchildren. As a community and from one generation to the next, we are accomplishing amazing things. I hope you will join us.
To learn more, visit the Highway of Heroes Tree Campaign online.
Next, read this letter from a Canadian soldier that explains the sacrifice of veterans everywhere.










 DIY Ways To Remove Kitchen Smells" width="295" height="295" />
DIY Ways To Remove Kitchen Smells" width="295" height="295" />


