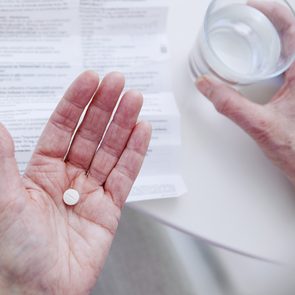
Here’s Why Doctors Are Prescribing Placebo Pills

It may not be active medicine, but the so-called placebo effect can provide real relief.
Michael Wharrad held the envelope in his hands, certain of what the paper inside would tell him. A decade ago, the 72-year-old, a former investment banker who lived in Kent, England, had been diagnosed with Parkinson’s disease. For a year, he had participated in a drug trial at London’s National Hospital for Neurology and Neurosurgery. Researchers were testing whether a medication approved to treat type 2 diabetes could also treat Parkinson’s symptoms. Every day, Wharrad had received a dose of either the drug or a placebo, but he never knew which.
During the trial, Wharrad thrived. His joints ached less, and he could get up from a chair more easily and take walks around the block. He also noticed that his memory seemed stronger. Friends and family commented on his obvious improvement. “My wife and I were convinced I was taking the drug,” he says.
But at his end-of-trial meeting with one of the researchers—who also didn’t know whether Wharrad had been on the drug or not—he was delivered a surprise. When he opened the envelope to find out what he’d been taking, he saw the word “placebo.”
“I was speechless,” he says. “I had been feeling so much better.”
How Placebos Work
A placebo can be a sugar pill, a saline injection or a glass of coloured water: inert treatments that shouldn’t produce a physiological response. But they often do; Wharrad’s case is not unusual. In fact, placebos are increasingly proving to be more powerful than active drugs in trials—and they may just be the key to reducing our dependence on medications.
The so-called placebo effect happens when the brain convinces the body that a fake treatment is authentic, which stimulates relief. The medical community has long been aware of this phenomenon, but in the last 50 years, neurologists began examining the molecular mechanisms and pathways at play when a mock treatment creates real healing. To a large extent, it’s still a mystery, but scientists have confirmed that simply perceiving that you’re being treated affects the part of our brain that processes symptoms.
Since the body-brain response that controls the placebo effect is neurological, they work best for conditions controlled by the neurological system, such as pain, irritable bowel syndrome, depression and Parkinson’s disease. Placebos can’t change things like a viral infection; they won’t lower your cholesterol, shrink a tumour or reduce a cold’s duration.
When they do work, expectations play a significant role: if you think a pill can cure you, it is more likely to do so. In a Lancet review of placebo studies, researchers described a case where post-surgery patients were given morphine for pain. For some, the medication was delivered secretly with a hidden pump, while others received it from a physician who explained that it would make them feel better. The patients expecting the drug and its positive effects experienced far greater pain reduction than those who were unaware they had received it.
Just As Good As Active Drugs?
Placebos can also work as a result of “pharmacological conditioning”— when clinicians teach a patient how to respond to a placebo by first administering an active treatment. According to Luana Colloca, a professor at the University of Maryland’s department of anesthesiology, this can result in the strongest placebo effect. Studying this phenomenon across a range of conditions, Colloca has observed via fMRI scans and other objective measurements that placebos use the same neurological pathway of the brain as the medication did. “The placebo response is like a pharmacological memory,” she says, explaining that it’s similar to a trauma response, where the brain reacts in a certain way to a traumatic event and then is later triggered to replicate that same response.
This specificity means that placebos for depression activate serotonin, and those replacing painkillers reduce activity in the brain centres responsible for pain while activating the opioid systems, or pleasure centres. In other words, your brain is tricked into generating a drug response.
Placebos are so powerful that they’re affecting the chances that a medication will be approved for use. To prove a drug works, scientists must show that it performs significantly better than a placebo in a randomized, double-blind, placebo-controlled trial (in this type of trial, neither the researchers nor the participants know who is receiving what). Over the past two decades, scientists and drug companies have noticed that placebos are helping patients so much that some drugs can no longer outperform them—not because the drugs are less effective but because the mind’s power over the body seems to be growing. In fact, a 2021 Danish meta-analysis of 180 drug trials showed that, in total, over half of the treatment effect could be attributed to a placebo.
This increase is not well understood, according to Lene Vase, a professor of neuroscience and psychology at Denmark’s Aarhus University, but it’s presenting a problem for drug companies.
“Some drugs that were approved in the past would not beat a placebo today,” she says.
Currently, this phenomenon seems to be strongest in the United States. Testing results for the drug lumateperone provide a typical example. In 2019, pharmaceutical company Intra-Cellular was on the verge of a major development for the treatment of bipolar depression; it had performed well in earlier trial phases, and the company’s scientists were expecting success. Yet in the American arm of the trial, patients who received the drug and those who received the placebo both experienced significant improvement. When Intra-Cellular released its findings showing the drug had failed to consistently outperform a placebo in part of its trial, its stock dropped 22 per cent—although it was later approved by the FDA due to successes in other countries.
Dr. Jeffrey Mogil, who studies pain at McGill University, published a paper in 2015 showing that the placebo effect has increased, especially in the United States. He posits that, because American trials are often more expensive and hosted in nice clinics, the patient is conditioned to believe the medication must work. Neuroscientist Alexander Tuttle, a co-author of the McGill study, hypothesizes that advertising also plays a part: Americans who view ads depicting patients helped by pharmaceuticals could also be more likely to believe the pill they take in a trial will heal them.
(The U.S. is the only country besides New Zealand that allows pharmaceutical companies to advertise prescription medication directly to consumers.)
A Case For Transparency
Placebo research is now its own area of study, and experts say we should harness the strategies that generate the most powerful placebo effects in drug trials and incorporate them into clinical treatments. It may sound unlikely, but in some cases, placebos work even if you know you’re taking one.
The effectiveness of “open-label placebos”—sometimes also called “pure” placebos—has been shown in numerous studies. In one published in the journal PLoS One, a team of researchers gave patients with irritable bowel syndrome inactive pills labelled “placebo.” Those patients experienced a 60 per cent improvement in their condition, while those receiving no treatment only improved 35 per cent. “The key ingredient to successful treatment with a placebo is honesty, not tricks,” asserts Dr. Ted Kaptchuk, the director of Harvard University’s Program in Placebo Studies and Therapeutic Encounter, who led the study.
Already, without telling patients, some doctors prescribe an active treatment—a vitamin or antibiotic, for example—that they know will likely not treat their ailment but may generate a placebo effect. In fact, a 2018 international review of studies from 13 countries found that up to 89 per cent of physicians reported using placebo treatments at least once a month. Doctors surveyed said they do this to treat non-specific complaints or to satisfy patients’ demand that something be prescribed. The hope, then, is that open-label placebos could replace this ethically murky practice. “Use of open-label placebos would reduce the amount of medication we use for common conditions,” adds Kaptchuk.
Although knowingly taking a faux treatment won’t be for everyone— Wharrad, for instance, isn’t convinced he would have experienced the improvements in his Parkinson’s symptoms had he been aware he was taking a placebo—one 2016 American study published in BMJ Open found that up to 85 per cent of the 853 respondents felt it was acceptable for doctors to treat with open-label placebos in various scenarios.
The Future of Placebo Research
In 2017, more than two dozen international placebo researchers gathered in the Netherlands to begin developing official recommendations around the use of open-label placebos, some of which were published last year. They include informing patients about placebo effects and fostering warm, trusting and empathetic patient-doctor relationships.
This potential transformation in medicine has already changed the lives of some patients who’ve participated in open-label placebo studies. Troy Mack, a 57-year-old Baltimore resident, had been suffering for two decades from intense pain in his face, neck and jaw from temporomandibular (TMD) joint disorder. When researchers at the University of Maryland, including Colloca, announced a study of an experimental TMD treatment, Mack was told that, based on his medical history, he could be a good placebo responder.
That prediction turned out to be correct. After just a week of knowingly taking a placebo, most of Mack’s jaw pain had disappeared. His face felt more relaxed, and the joint no longer cracked when he yawned. He was finally experiencing relief. “If I could get a long-term prescription for this, I would take it,” he says.
Now that you know why placebos work, find out if your supplements are doing more harm than good.